Introduction: MF is a clonal stem cell disorder characterized by progressive bone marrow failure, extramedullary hematopoiesis, and debilitating constitutional symptoms. Approximately 60% of pts develop anemia within a year of diagnosis; pts who are transfusion dependent have worse survival and quality-of-life outcomes (Tefferi A, et al. Mayo Clin Proc 2012;87:25-33). Treatment for MF-associated anemia remains critically unaddressed, with no approved therapies available. Luspatercept is a first-in-class erythroid maturation agent that has been shown to increase hemoglobin and reduce transfusion burden in pts with myelodysplastic syndromes (Fenaux P, et al. N Engl J Med 2020;382:140-51) and β-thalassemia (Cappellini MD, et al. N Engl J Med 2020;382:1219-31). Pts have also achieved multiple response episodes with luspatercept (Fenaux P, et al. Blood 2019;134:841). We previously reported findings from the ongoing open-label, phase 2 ACE-536-MF-001 trial evaluating luspatercept in pts with MF-associated anemia (Gerds AT, et al. Blood 2019;134:557). Here we report updated study results, focusing on response in pts requiring RBC transfusions.
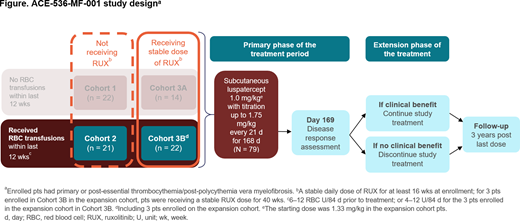
Methods: 79 pts with MF and anemia were enrolled (Figure). Of these, 43 were requiring RBC transfusions, defined as 6-12 U/84 d prior to treatment, or 4-12 U/84 d for the 3 expansion cohort pts. Pts in Cohort 3B received a stable ruxolitinib (RUX) dose for ≥ 16 wks (40 wks for expansion cohort pts), whereas Cohort 2 pts did not (Figure). Pts in Cohort 3B received a median RUX dose of 20 mg/d (range 5-40) for a median of 73 wks (range 29-359) prior to treatment.
Pts received luspatercept every 21 d at a starting dose of 1.0 mg/kg (1.33 mg/kg for 3 expansion cohort pts in Cohort 3B), with doses escalating up to 1.75 mg/kg; 81% and 50% of pts in Cohorts 2 and 3B escalated to 1.75 mg/kg. Primary endpoint for pts receiving transfusions was RBC transfusion-independence (RBC-TI) for ≥ 12 consecutive wks within the first 24 wks on study. However, as responses were observed that did not fall within the 24-wk response window, additional assessments were conducted for the entire treatment duration for RBC-TI and ≥ 50% decrease in RBC transfusions (minimum 4 RBC U decrease) from baseline. Intent-to-treat data were analyzed as of March 29, 2020.
Results: Pts in Cohorts 2 and 3B received a median of 8 cycles of luspatercept (range 1-39), for a median (mean) duration of 24 (31) and 23 (39) wks, respectively. Pts in Cohorts 2 and 3B received a median of 2.7 RBC U/28 d (range 1-5) and 2.7 RBC U/28 d (range 2-4), respectively. For the primary endpoint, 2/21 (10%) and 6/22 (27%) pts receiving transfusions in Cohorts 2 and 3B achieved RBC-TI over any consecutive 12 wks during the first 24 wks. Median time to start of first RBC-TI response period in Cohorts 2 and 3B was 1.5 days and 37 days; median duration of response was 49 wks (range 16-82) and 42 wks (range 12-111), respectively.
When assessing the entire treatment period, 4/21 (19%) and 8/22 (36%) pts in Cohorts 2 and 3B achieved RBC-TI ≥ 12 wks, respectively. 25% of the RBC-TI responders in both cohorts experienced 2 separate episodes of RBC-TI ≥ 12 wks.
Median cumulative duration of all individual ≥ 12-wk response episodes of RBC-TI was 59 wks (range 24-82) in Cohort 2 and 55 wks (range 12-116) in Cohort 3B.
8/21 (38%) and 10/22 (46%) pts in Cohorts 2 and 3B achieved a ≥ 50% reduction in RBC transfusion burden (minimum ≥ 4 U reduction) over 12 wks; of these, 38% and 20% of responders in Cohorts 2 and 3B experienced 2 separate ≥ 12-wk response episodes, and 1 pt (13%) in Cohort 2 experienced 3 separate responses.
4/15 (27%) and 8/14 (57%) pts in Cohorts 2 and 3B who reached 24 wks of treatment achieved clinical benefit and therefore continued luspatercept on an ongoing basis.
Safety is reported for all 79 pts on study. 5 (6%) pts had grade 3-4 treatment-related adverse events (AEs); these AEs were diarrhea (n = 2), dehydration (n = 1), and hypertension (n = 3). There were no treatment-related deaths. Treatment-related AEs (any grade) occurring in ≥ 5% of pts were hypertension (13%), bone pain (9%), and diarrhea (5%). 8 (10%) pts had ≥ 1 AE leading to discontinuation. 16 (20%) pts remain on treatment.
Conclusions: These longer-term findings suggest durable activity of luspatercept in pts with MF-associated anemia. In addition, a quarter of pts receiving transfusions achieved more than one ≥ 12-wk episode of RBC-TI response with luspatercept. The incidence of grade 3-4 AEs with luspatercept was low.
Gerds:Roche/Genentech: Research Funding; Celgene: Consultancy, Research Funding; Sierra Oncology: Research Funding; AstraZeneca/MedImmune: Consultancy; Incyte Corporation: Consultancy, Research Funding; Pfizer: Research Funding; Apexx Oncology: Consultancy; CTI Biopharma: Consultancy, Research Funding; Gilead Sciences: Research Funding; Imago Biosciences: Research Funding. Vannucchi:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees. Passamonti:Novartis: Speakers Bureau; BMS: Speakers Bureau; Roche: Other: Support of parent study and funding of editorial support. Kremyanskaya:Constellation Pharmaceuticals: Research Funding; Incyte Corporation: Research Funding; Bristol Myers Squibb: Research Funding; Protagonist Therapeutics: Consultancy, Research Funding; Astex Pharmaceuticals: Research Funding. Gotlib:CTI Biopharma: Research Funding; Kartos: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Roche: Honoraria, Research Funding; BMS: Research Funding. McCaul:BMS: Speakers Bureau; Seattle Genetics: Speakers Bureau; Karyopharm: Speakers Bureau; Incyte: Speakers Bureau. Ribrag:argenX: Research Funding; pharmamar: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; servier: Consultancy; nanostring: Honoraria, Membership on an entity's Board of Directors or advisory committees; gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; AZD: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other; Infinity: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Honoraria, Membership on an entity's Board of Directors or advisory committees; gustave roussy comprehensive cancer center: Current Employment; EPZ: Honoraria, Membership on an entity's Board of Directors or advisory committees; epizyme (EPZ): Research Funding. Mead:Novartis: Consultancy, Honoraria, Other: travel, accommodations, expenses, Research Funding, Speakers Bureau; Celgene/BMS: Consultancy, Honoraria, Other: travel, accommodations, expenses, Research Funding; Abbvie: Consultancy; CTI: Consultancy; Gilead: Consultancy. Harrison:Roche: Honoraria; Gilead Sciences: Honoraria, Speakers Bureau; Incyte Corporation: Speakers Bureau; Janssen: Speakers Bureau; CTI Biopharma Corp: Honoraria, Speakers Bureau; Sierra Oncology: Honoraria; Celgene: Honoraria, Research Funding, Speakers Bureau; Shire: Honoraria, Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau; AOP Orphan Pharmaceuticals: Honoraria; Promedior: Honoraria. Mesa:Sierra Oncology: Consultancy; LaJolla Pharmaceutical Company: Consultancy; Novartis: Consultancy; Incyte: Research Funding; CTI BioPharma: Research Funding; Genentech: Research Funding; AbbVie: Research Funding; Promedior: Research Funding; Bristol Myers Squibb: Research Funding; Samus Therapeutics: Research Funding. Kiladjian:AbbVie: Membership on an entity's Board of Directors or advisory committees; AOP Orphan: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Barosi:Bristol Myers Squibb: Consultancy. Gerike:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Shetty:BMS: Current Employment, Current equity holder in publicly-traded company. Pariseau:Bristol Myers Squibb: Current Employment. Miranda:Bristol Myers Squibb: Current Employment. Schwickart:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Giuseppi:BMS: Current Employment, Current equity holder in publicly-traded company. Zhang:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Backstrom:Acceleron Pharma: Current Employment, Current equity holder in publicly-traded company; BMS: Current equity holder in publicly-traded company. Verstovsek:AstraZeneca: Research Funding; Roche: Research Funding; CTI Biopharma Corp: Research Funding; Genentech: Research Funding; Incyte Corporation: Consultancy, Research Funding; NS Pharma: Research Funding; Novartis: Consultancy, Research Funding; PharmaEssentia: Research Funding; ItalPharma: Research Funding; Sierra Oncology: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Blueprint Medicines Corp: Research Funding; Promedior: Research Funding; Gilead: Research Funding; Protagonist Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.